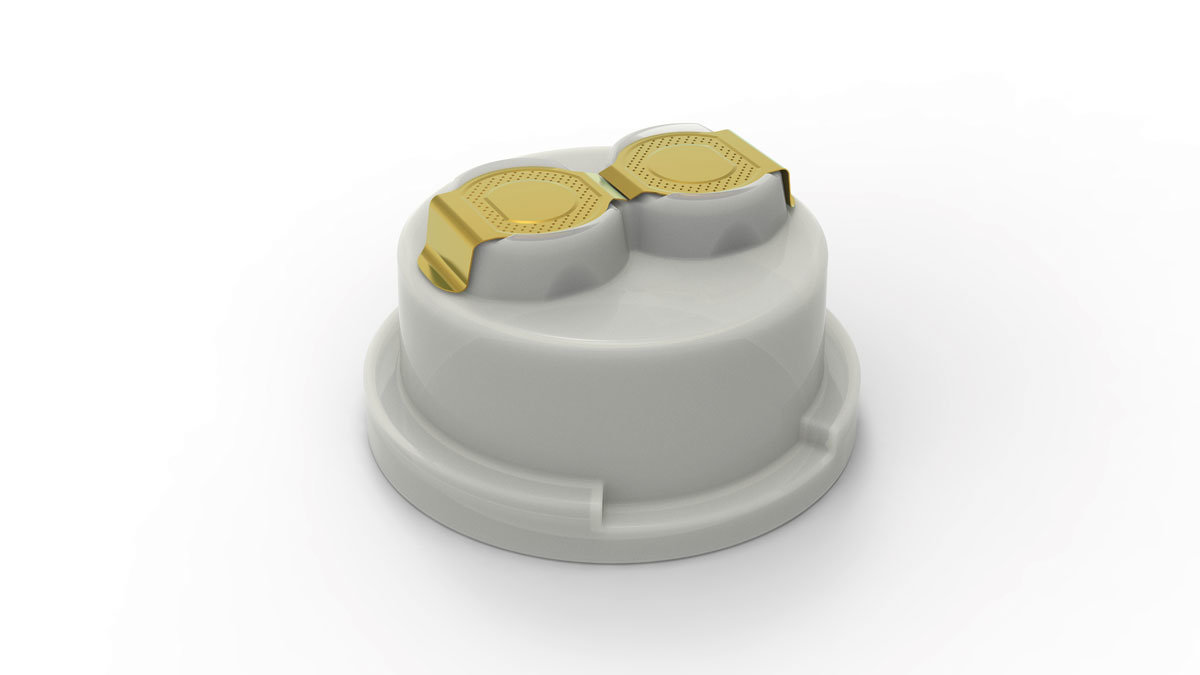
DoubleA-Cap®
Pure and clean quality
World-class closure cap for infusion containers manufactured using the BFS (Blow-Fill-Seal) process.
The DoubleA-Cap® is produced in qualified clean rooms class GMP D. The caps for BFS applications are made of approved medical grade materials and comply with the DIN EN ISO 15759 and DIN EN ISO 8871-5 standards.
Convincing features:
- Suitable for all IV containers with standard EuroHead
- Available for both PP and PE containers
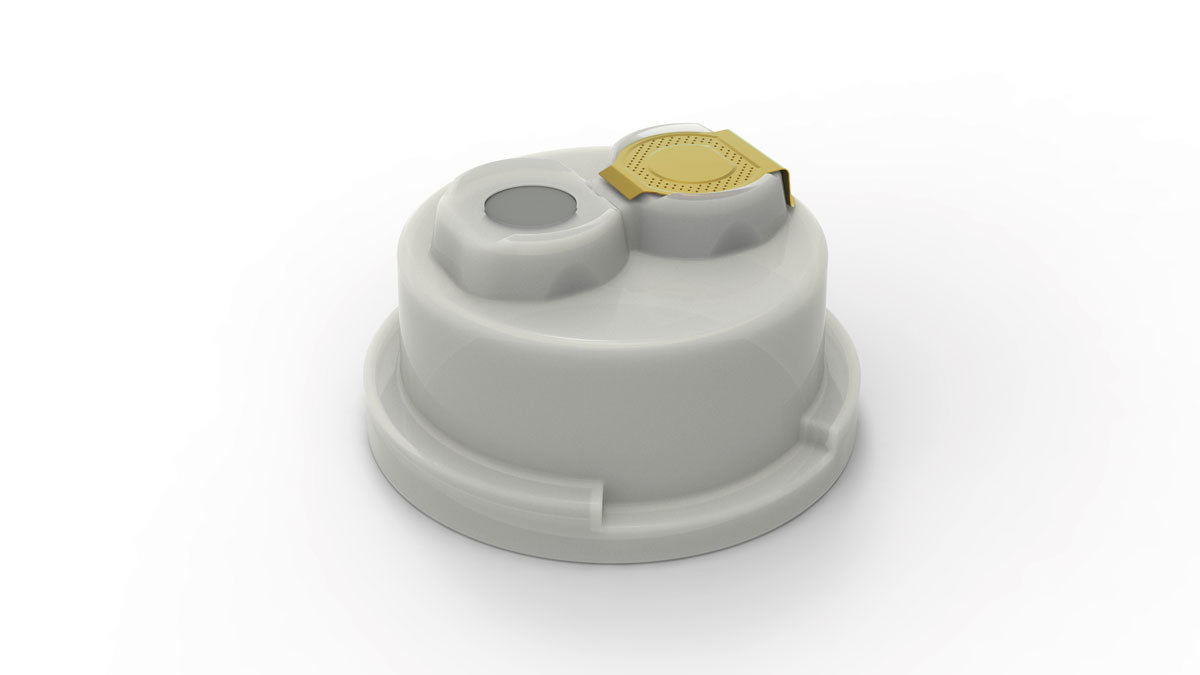
- Cap is oriented on bottle
- Compatibility with existing filling and capping lines
- Material usage lower than in existing products
- Two separate, equivalent ports for IV sets and cannulas
- Each port can be easily and individually opened while the other remains sealed and germ-free
- Safe and convenient access: low penetration and high retention force
- Excellent sealing and resealing properties
- Cap and elastomer seals are inseparably combined inside the cap preventing a secondary leak-path around the seals
- Low coring and fragmentation during spike and needle insertion
Pharma meets technology
Through a combination of pharmaceutical and technology know-how, a perfectly sophisticated closure cap, the DoubleA-Cap®, was developed. All information about the DoubleA-Cap® in our flyer.
Pharma products
We offer our pharmaceutical industry customers a fully integrated spectrum of solutions with maximum safety for a demanding production.